 01-30-2025
01-30-2025
Mission Need
The National Institute of Health’s (NIH) National Cancer Institute’s Center for Biomedical Informatics and Information Technology (NCI CBIIT), in conjunction with the Frederick National Laboratory for Cancer Research, currently operated by Leidos Biomedical Research Inc., is dedicated to driving progress in biomedical informatics and data science in cancer research. Managing cancer-related common data elements (CDEs) and associated metadata correctly is essential to boost research efforts, enable data sharing and guarantee interoperability within the health IT ecosystem. However, researchers and health care providers still face obstacles when trying to integrate and apply disparate data sources for cancer research and patient treatment. There was a necessity to improve the curation, harmonization, access and use of CDEs and metadata in the Cancer Data Standards Registry and Repository II (caDSR II) across the clinical trial and cancer research community to overcome these challenges.
Solution
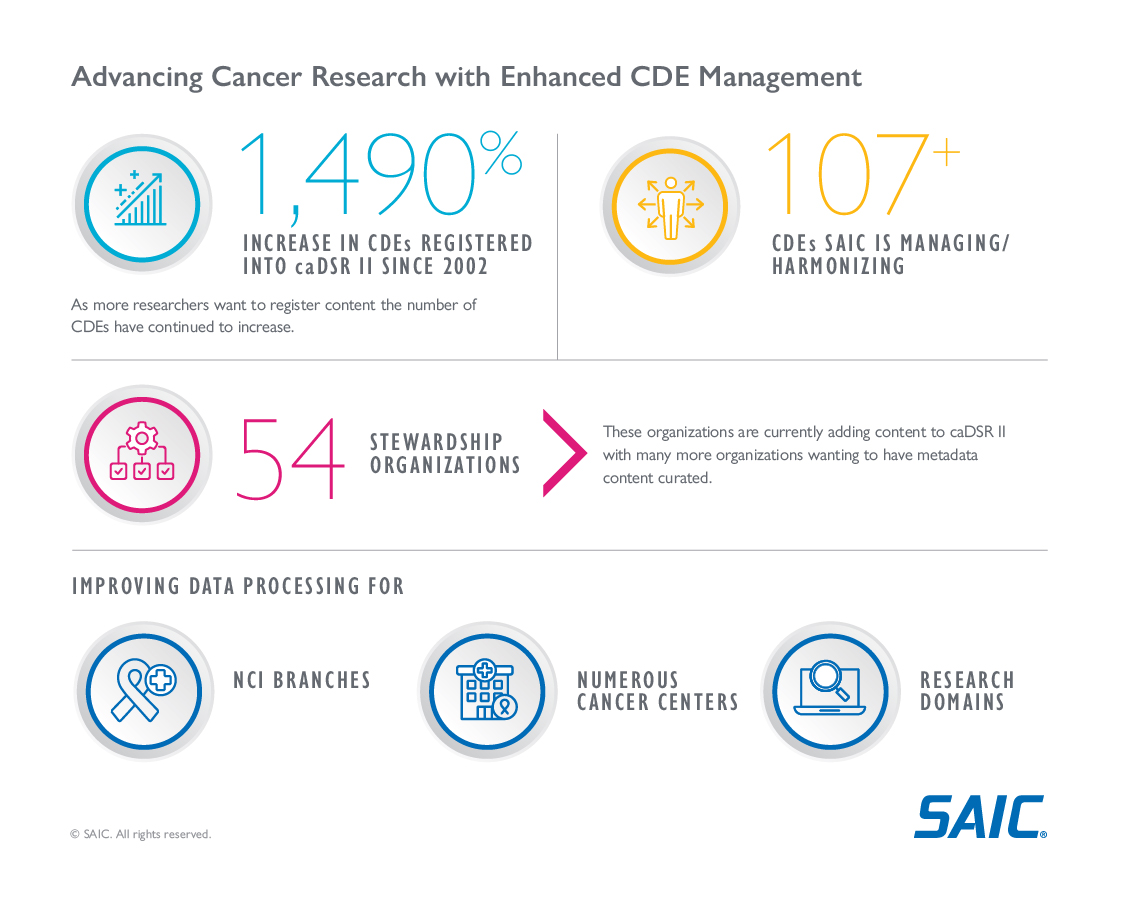
SAIC worked for the NCI CBIIT to provide mission expertise and services to monitor, manage and maintain the collection, curation and use of CDEs and metadata. By improving the quality and accessibility of data, we helped empower researchers and clinicians to advance research, accelerate discovery, collaborate with cancer researchers across the globe and improve patient outcomes.
Click image to enlarge
How We Helped
- Played a key role in the comprehensive process of collecting, analyzing and mapping of CDEs and associated metadata to ensure accuracy and relevance.
- Assisted with the integration of data into a modern, scalable framework designed for ongoing curation and harmonization to enhance data quality and consistency across cancer research entities and support the evolving needs of cancer data and analysis.
- Developed and provided specialized training and mentorship initiatives so stakeholders have the knowledge and skills necessary for effective data management.
- Supported the creation and maintenance of governance models that ensure adherence to data standards while allowing for agile updates in response to new scientific and technological advancements.
- Fostered strategic partnerships with various research and health care institutions to broaden the impact of harmonized CDEs and metadata on cancer research and patient care.
- Implemented a metrics-driven approach to track harmonization efforts, inform stakeholders of progress and identify areas for further enhancement.
Mission Impact
This harmonization initiative enhances the quality, access, use and exchange of health data among research entities and systems. Streamlining and improving efficiencies in data management and research processes promotes greater collaboration among researchers and enhances the coordination of patient care. The availability of high-quality, reliable and accurate data provides a strong foundation for scientific research and discovery.
By strengthening the capabilities of the NCI CBIIT through targeted training programs that elevate expertise in managing complex data sets and updating governance structures to ensure that data standards evolve in step with new research developments, the NCI CBIIT is uniquely positioned to lead and innovate in clinical trials and cancer research.
The success of this initiative has piqued the interest of other NIH institutes and centers, as well as various federal agencies. They are noticing the advantages of adopting a centralized metadata management system, and many are eager to begin using the caDSR II system and tools within their own organizations.
The views, opinions or findings contained in this report are those of the author(s) and should not be construed as an official Government position, policy or decision, unless so designated by other documentation.
Learn more about SAIC's federal civilian innovations


